Saturday, September 14, 12024 Human Era (HE)
Wow! A second Whey-ah-wichen Whipper (my first was last year) signals a full season of S’ólh Téméxw stand up paddleboard (SUP) racing. S’ólh Téméxw, pronounced “soul tow-mock” means “our land” or “our world” in Halkomelem. S’ólh Téméxw is one of a long list of anticolonial (and colonial) names that have been suggested for a potential renaming of British Columbia (a suggestion that is all too unlikely to happen, but a worthwhile thought experiment nonetheless). Names and renamings are a fitting opening for this narrative given the historical names and name changes associated with the launch site for this posts topic of interest, the Whey-ah-wichen Whipper.
The Whipper launches from The Park Formally Known as “Cates Park,” the official title now being Cates Park/Whey-ah-wichen. Located in səl̓ilw̓ət (Indian Arm – see I told you this would cover a colonial name theme) at Roche Point, the point was named for Lieutenant Richard Roche in 11948 HE. “Cates Park” was established in 11951 HE, after the land was donated by the Cates family in memory of the founders of the Cates Towing Company, Captain Charles Henry Cates and his wife Mary Wilhelmina Barrow. The indigenous component of the park’s renewed name, “Whey-ah-wichen,” is from the hən̓q̓əmin̓əm̓ (Hun’qumyi’num) language and translates to “facing both directions” and “facing the wind.”
Whey-ah-wichen was an ancestral village that was believed to be a winter site prior to 11846 HE. After that time, due to the introduction of European diseases, the site is reported to have transitioned to a spring and summer village.
Today, Vancouver’s North Shore Tourism touts the park as an excellent outdoor space. It has six kilometres of waterfront walking trail that winds past and through beaches and coastal rainforest, ‘perfect for low tide beachcombing.’
I Know What You Did Last Summer
To read my account from last years Whipper, click here. Be warned, it is a four-part saga (minus the heroic achievement), ranging from topics of history and sports hydration to the benefits of a warm-up to sex in sport and performance-enhancing substances to a race recap and my views of the distinction between physical activity and exercise.
Compare if You Dare
The Whipper offers three race lengths. A short, 7-kilometre, a 12-kilometre mid-distance, and a long distance 18-kilometre (see below). Since I was persuaded to do the 18-kilometre course last year, I felt compelled to do the same course this year. I was curious to compare my race results, acknowledging the caveat that course conditions could be inconsistent.

Paddlesports, unlike my previous sporting endeavours (e.g., basketball or hand/football), have the confound that climate conditions like wind and water currents affect performance. Multi-directional team sports (i.e., basketball, foot/football, and hand/football), however, are much more dependent on your competition’s performance than the environmental conditions, as the external variable. In other distance or time-based race events, environmental conditions exert an effect, but often less so than paddlesports, particularly SUP. Under these circumstances, you can compete against the course [i.e., yourself (and the competition)] more directly. Given my transition from physical activity to exercise, I was curious how I would compare both counts, that is, contra- course and competition.
PREP: Poor Result Expectation – Preparations
My long-term preparations started after last year’s Whipper. My personally perceived poor finish resulted in a re-evaluation of my physical activity practice. If I wanted to do better in competition (which I did), my present practice of purely physical activity needed to be progressed to proper exercise (i.e., goal-directed physical activity).
Leading into this year’s SUP season, I had amassed an array of exercise conditioning sessions. It served me well for my Jericho Wavechaser races. The remaining question was, what effect would my improved fitness have on this year’s Whipper?
Lab-Based Shenanigans
In addition to my re-entry into exercise, I was also exploring additional aspects of exercise science. Way back in one of my undergraduate Human Kinetics courses [in the early 12000s (HE)], on high-performance conditioning, I was introduced to the idea of sodium bicarbonate (NaHCO3) loading (or bicarb loading – not to be confused with carbohydrate loading or carb loading). At the time, it was discussed as a niche/fringe approach of a legal ergogenic aid/performance-enhancing substance, much like caffeine. From memory, it was presented as a proven way to improve sprint performance, with the caveat that it could blow up in your face, literally. Taking sodium bicarbonate was often associated with gastrointestinal distress.
Brief Notes on Blood Buffering
How does sodium bicarbonate (yeah you got it, the everyday kitchen item baking soda) improve athletic performance you ask? Good question. First things first, let’s chat electrolytes, then we will circle back to our discussion about blood buffering.
The Less Famous Electrolyte
Electrolytes are minerals in your body fluids that carry an electric charge (i.e., they are ions). The etymology of electrolyte derives from the Ancient Greek prefix ēlectro-, originally meaning amber, but in modern contexts, it is more in reference to electricity, with lytos meaning “able to be untied or loosened.” A fitting name for their function of untying electricity in the body. It is by way of this electrical potential that electrolytes function, exerting their influence on nerve excitability, endocrine secretion, membrane permeability, buffering body fluids (i.e., pH), and fluid balance.
The Usual Suspects
When it comes to electrolytes, many of us are familiar with the usual suspects, sodium (Na+) and potassium (K+), the big name players you hear about in sports drinks. But there are four other key players that make up the big six electrolytes [chloride (Cl–), calcium (Ca2+), phosphate (HPO42-), and bicarbonate (HCO3–)], in addition to a host of other lesser known electrolytes (again, anything carrying a charge counts). So, HCO3– fits the criteria, a fact I am slightly embarrassed to admit I did not explicitly put together until writing this post (for some reason my brain defaulted to thinking electrolytes needed to be elements/atoms and not compounds). The main function of HCO3– in the body is to help with acid-base balance (it is a blood buffer). Somewhere, someone had the idea that HCO3– could help with the increased acidosis (i.e., a lower pH) that comes with exercise and increased metabolism.
The knowledge that NaHCO3 could benefit exercise performance goes as far back as the 11930s HE. In my KIN class, the simple explanation was that the HCO3– liberated from NaHCO3 in an aqueous environment buffered the blood. Let’s decompact that a bit more…
Blood Buffer Basics
As mentioned, during cellular respiration, metabolism moves the body toward acidosis in a dose-dependent manner (i.e., higher metabolism equals greater acidity – BUT IT’S NOT LACTATE THAT IS DOING IT – at least not directly). The increased acidity is the result of both the breakdown of fuel substrates resulting in CO2 production, as well as the hydrolysis of adenosine triphosphate (ATP) molecules [it is the hydrolysis of ATP to liberate usable energy that is the source of extra protons (i.e., acid), contrary to the historic view]. Carbon dioxide formed during cellular respiration is transported in the blood to be expelled from the body via the lungs. There are three main ways that CO2 is transported in the blood, with the majority being moved as HCO3–. Essentially, CO2 diffuses into the blood and combines with water in a chemical reaction catalyzed by the enzyme carbonic anhydrase to form carbonic acid (H2CO3). Carbonic acid then dissociates into a bicarbonate anion (HCO3–) and a proton (H+) to be moved to the lungs where the reverse reaction occurs. Below is the reaction on the cellular respiration side of the system (though this is happening in the lung cells too, just to a lesser degree).
CO2 + H2O ➔ H2CO3 ➔ H+ + HCO3–
But let’s get back to bicarbonate and blood buffering…
Back to Bicarb
If you want an in-depth, thorough explanation, check out this position stand paper, “International Society of Sports Nutrition position stand: sodium bicarbonate and exercise performance,” from the Journal of the International Society of Sports Nutrition. Or check out this video summary for a medical/non-exercise explanation. In exercise, the CO2 levels in the blood are rising due to increased metabolism and pH is decreasing (i.e., increased H+ ions) due to ATP hydrolysis.
For the less inclined (🤓), here are the Coles Notes of what happens when you consume NaHCO3.
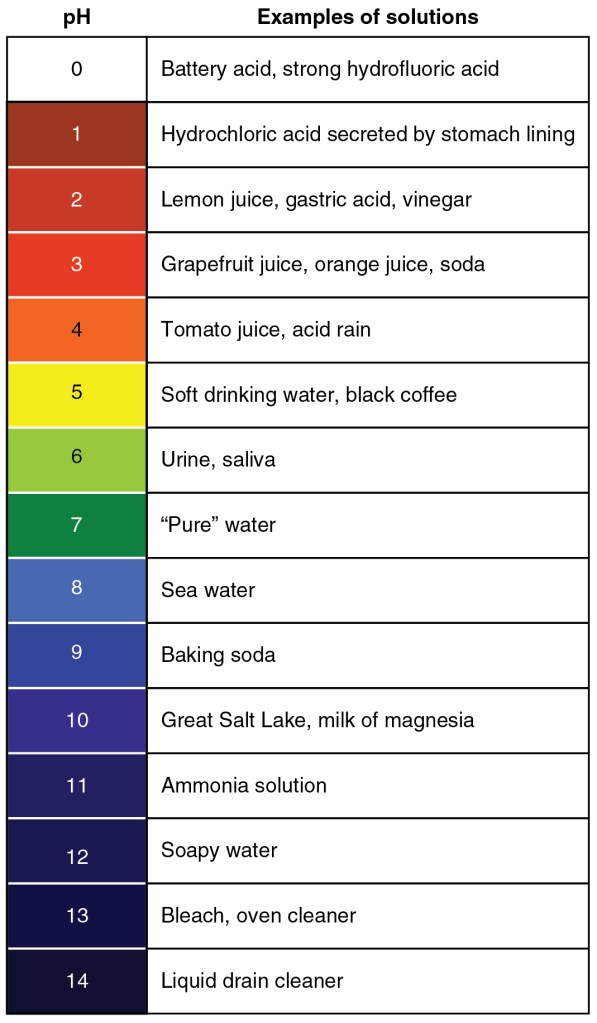
As a primer/refresher, the table below lists the acidity of various solutions. In particular, note hydrochloric acid (HCl) at a pH of ~1 and baking soda (NaHCO3) at ~9.
First off, NaHCO3 rapidly dissociates on contact with an aqueous solution into its constituent ions [sodium (Na+) and bicarbonate (HCO3–)].
NaHCO3 ➔ HCO3– + Na+
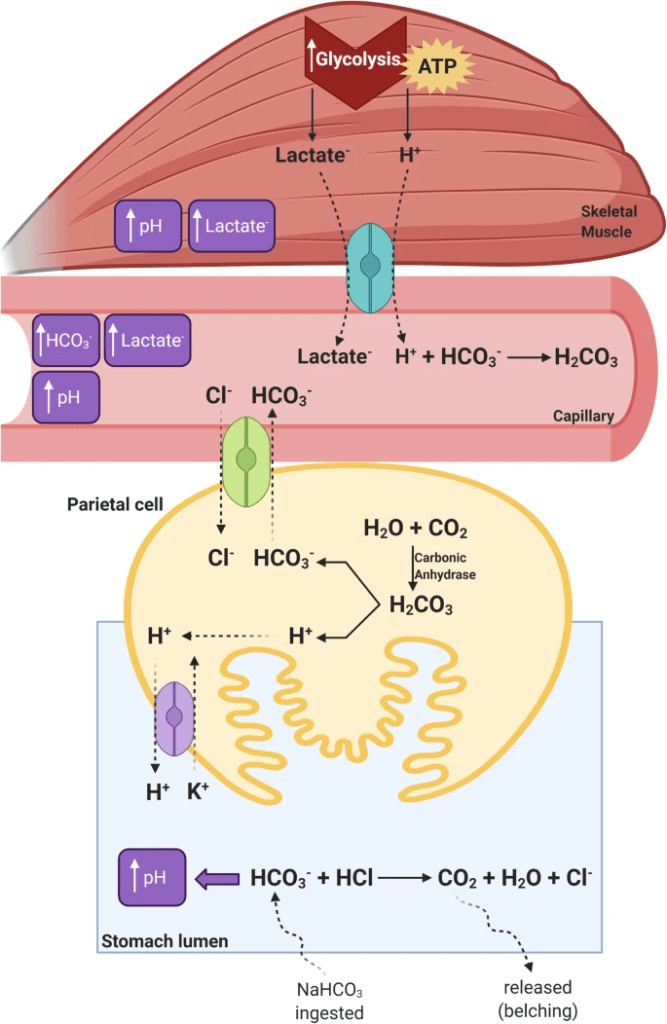
Given that one of the first places this happens is in the acidic environment of the stomach, there is a neutralization reaction. Recall that from high school biology that the acid secreted by the cells lining your stomach is HCl and keeps the pH in the stomach around 1.5 to 3.5. The HCl (acid) is neutralized by the HCO3–(base) to eventually form water and a salt [in this case sodium chloride (NaCl) or table salt, but in its dissociated ionic form of sodium (Na+) and chloride (Cl–) ions]. There is an intermediate step where carbonic acid (H2CO3) is formed but then reacts with the enzyme carbonic anhydrase to form carbon dioxide (CO2) and water (H2O). The eventual fate of the ingested NaHCO3 as CO2 and H2O slightly alkalizes the stomach (i.e., increasing the pH) as there is now less HCl in the mixed stomach juices (you’ll recognize the second step as the reverse of the ‘CO2 off-loading’ equation from before).
HCl + HCO3– + Na+ ➔ H2CO3 + Cl– + Na+
H2CO3 ➔ CO2 + H2O
In the stomach, the CO2 that is formed becomes volatile, resulting in stomach gas. This explains some of the common side effects of bicarbonate loading, bloating and belching, and is the most likely reason that bicarbonate loading is not more prevalent in sport. It is a risky trade-off to performance enhancement if you have a belly full of bubbling gas [see the bottom of the image below which shows a gastric parietal cell and the stomach lumen (i.e., the inside of the tube) for a schematic representation of the process].
Back in university, the threat of gastrointestinal discomfort was enough to scare me off of experimenting with the protocol, but the potential performance pearl was stored away as a novelty hack.
Where the Magic Happens
Where the real magic happens is that some of the ingested NaHCO3 will make it into the bloodstream as HCO3–. There, it is able to work, increasing the blood’s buffering and ultimately the body’s tolerance to the formation of acid that accompanies higher levels of exercise metabolism.
Converging Coincidences
Recently, my workplace started stocking Lactigo, ‘a topical muscle recovery and performance sports gel.’ Aside from being a topical application of menthol, the other likely mechanism of action for this product is as a buffering agent. Lactigo contains carnosine, which is reported to work as a muscle buffering agent. So buffering agents were already beginning to blossom in the forefront of my brain.
Add in the burgeoned popularity of biohacking along with the recent resurgence of baking soda doping making headlines, and I hearkened back to the buffering beliefs of my body science bachelor’s degree. This timing also coupled with my ever steady shift towards more liberal views of drugs and performance hacks, along with a new found interest in endurance/work-based sports, so I was ready to attempt arming with Arm & Hammer.
Experimental Dabbling
But first things first, I had to figure out dosing, as the shortened adage attributed to the alchemist physician Paracelsus admonishes, ‘the dose makes the poison.’ Thankfully, the position stand previously mentioned along with this popular science post from Training Peaks covered the basics of dosing. For an acute dose, the evidence suggests approximately 0.2 to 0.3 g/kg of body weight are needed to improved muscular endurance. Next questions is how much baking soda is that?
Thankfully the article listed that, “grocery-store-variety baking soda from Arm and Hammer contains 0.6 grams of NaHCO3 per 1/8 teaspoon.” So basically, a teaspoon of baking soda is five grams of NaHCO3. The rough math for me was I needed about 25 grams of baking soda to get to 0.3 g/kg, or about five teaspoons. Mixed with water and slugged down it is a nasty concoction, but doable.
In the lead up to the race, I had completed a few successful session with bicarb on board. Perhaps it was placebo, but I perceived some performance gain, and definitely did not determine it to be detrimental.
With all that out of the way, let’s get back to the Whipper.
Registration/Check-In
Registration for The Whipper is a simple process available online through the Webscorer website. This year I was able to take advantage of the online race check-in since I had a bib number from my early season participation in a few TNR events. This meant I could show up a bit later on race day. A welcomed perk.
Wetter Weather
Unfortunately, the forecast for this year’s Whipper called for rain. Since I had been in səl̓ilw̓ət (Indian Arm) for the same weekend the past two years (see “SUP to Thywates Landing” and “Words on the Whey-ah-wichen Whipper” for details) with phenomenal weather, my expectation, like many others, was for warmth and sunshine. On race day, I heard several groups of competitors highlighting the difference in weather for this year. The consolation was that it was not cold, nor was it windy.
Meeting in the Rain
After arriving on the scene, I set up my kit and then brought my board down to the pre-race meeting area. At the time, it was not quite raining, but it was definitely threatening to. I found a spot under the awning to Wally’s Burgers in case the rain picked up. Ready for a meeting at the meat-thing.
There were several familiar faces from the paddling community that I made small talk with as we waited for the pre-race meeting to start. Though, of note, there was an absence of representation from the Jericho Wavechaser cohort. In the meanwhile, I also performed some dynamic movements as part of a warm-up, the benefits of which cannot be overstated, in my opinion.
As the pre-race meeting commenced, I was sure to pay attention to the race rules around drafting. Last year, my lack of attention left me lamenting the legalities of libertine labels for lee-sided laneway leads. The rules for this year were what my inattentive interpretation from last year concluded. Drafting between sexes was legal as long as the vessels matched categories. With no changes to the race course, the meeting was a refresher of the rules and route.
During the pre-race meeting, the rains opened up. I was happy to be under the cover of the meat thing for the meeting. But as predicted by a fellow racer from their reading of the weather radar, the rain was short-lived and had mostly ended by the time the meeting concluded. Post-meeting, I removed my additional water protection and warmth layers, then made my way to the Whey-ah-wichen waters for my paddle specific warm-up.

The səl̓ilw̓ət waters were calm, aside from the occasional boat wake, which we waterfolk were working to ride. In fact, the waves in the warm-up to the Whipper were wonderful. To the point that a fellow wavechasee, one who rides a surf ski, was sure to inform me that this was one of the best spots in the city to catch boat wakes as they become extra wavey. I took note and plan to head back there in the fall/winter to try my luck. And I will definitely be there next spring/summer when there will be guaranteed boat traffic volume.

2 thoughts on “Whey-ah-wichen Whipper Welcome Back”